Brandon Capital and Tenmile – Andrew and Nicola Forrest’s new healthcare technology investment company – have committed $10 million each to peanut allergy therapy company Aravax.
The $20 million funding will enable Aravax to start Phase 2 clinical trials of the immunotherapeutic it has developed to treat peanut allergy. The company plans to launch the trials early in the new year.
Melbourne-based Aravax was founded in 2015 with venture capital investment from Brandon Capital and the acquisition of technology from Alfred Health and Monash University. Brandon Capital and Tenmile’s new investment represents the start of the company’s Series B capital raising round.
Peanut allergy affects 1-2% of the global population but, according to Aravax, current treatment options are challenging. These are therapeutics, which seek to build tolerance by introducing patients to small exposures to peanut proteins, daily. The treatments need to be continued indefinitely and require onerous precautions to manage the risk of adverse reactions.
Aravax’s PVX108 therapeutic does not contain peanut proteins so does not pose a similar risk of side effects. PVX108 is designed to retrain the immune system to tolerate peanut allergens and is required to be taken monthly rather than daily.
Aravax chief executive Dr Pascal Hickey said: “PVX108 has revolutionary potential because we have engineered out the safety risks associated with current approaches. Furthermore, the precise action of PVX108 affords a vaccine-like dosing regimen which is expected to be more convenient than the daily dosing required for other therapies, likely resulting in better adherence.”
An application by Aravax for Investigational New Drug (IND) status for PVX108 has been approved by the US Food and Drug Administration (FDA) and the company has a Clinical Trial Notification in place with Australia’s Therapeutic Goods Administration (TGA). The Clinical Trial Notification allows Aravax to progress the Phase 2 clinical trial program in the US and Australia. The trial will start at several leading allergy clinics, and further capital will enable expansion of the study and work towards Phase 3.
Brandon Capital partner Dr Chris Smith said: “Aravax will have our continued support as it progresses into Phase 2 trials and beyond. Witnessing the positive data from pre-clinical studies and Phase 1, we’re excited about the potential of the therapy in creating a safer, more convenient treatment for people with peanut allergies.”
Executive chair of Tenmile, Dr Steve Burnell, said PVX108 was a novel peptide designed to address the life-threatening risk of peanut allergy but Tenmile was also excited about the potential for the platform technology to be used against other allergens.”
Tenmile was set up in July, with an initial allocation of $250 million, to invest in health technologies. The company has its headquarters in Perth with additional team members in Sydney and San Francisco. Tenmile operates under the umbrella of the Forrest family’s Tattarang Group investment business.
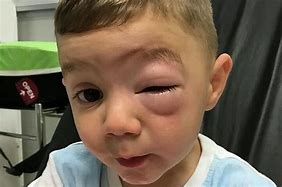
Image: Peanut allergy caused this swelling to the face of a two-year-old boy.

